Description
Description
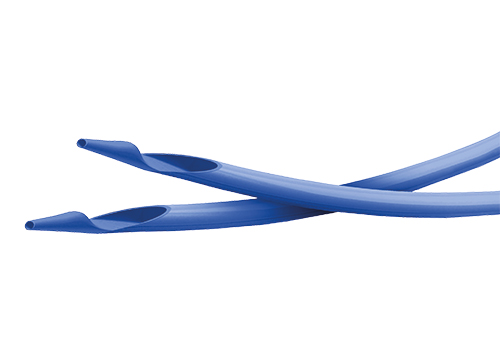
The sirolimus-eluting coronary stent angiolite is made from a cobalt chromium alloy called L605, coated with a mix of sirolimus and last generation of biostable polymers. The stent is pre-mounted on the delivery system that will allow implantation on the coronary lesion to treat thanks to the inflation of a balloon at the distal end of the catheter.
The stent is manufactured from a metal tube that is laser cut and subsequently subjected to various treatments that will give the surface a smooth, glossy finish. The stent design is based on a concatenation of cells in the circumferential direction that are connected to each other axially by means of links to obtain different longitudinal configurations. Moreover, the adjustment of the number of cells in the radial direction allows the stent to be expanded to different diameters. The result is an open cell design.
The stent delivery system is a rapid exchange balloon catheter also called RX, having a single lumen configuration on the proximal part and a coaxial double lumen configuration on the distal part. The catheter has an inflatable segment (balloon) in its distal end. The balloon is designed to achieve different diameters and lengths, engaging the stent in its different configurations and covering the range of lesions to be treated. Before positioning, the balloon is folded and the stent is compressed over it. There are radiopaque markers delimiting the length of the stent and to facilitate observation under fluoroscopy. When the balloon reaches the lesion and is inflated, the stent expands against the artery. Subsequently, the balloon is deflated and removed and the stent remains permanently implanted.
The distal part of the catheter is coated with a durable hydrophilic coating to minimize friction and improve its trackability.
The inflatable segment is coated with a homogeneous mixture of the drug Sirolimus (Rapamycin) and a biostable fluoroacrylate polymer yielding a drug dose of 1.4 µg/mm2.
Indications
- Patients with symptomatic ischemic heart disease due to “de novo” stenotic and re-stenotic lesions located in arteries with diameters from 2 mm to 4.5 mm
- Patients with occlusive disease due to acute myocardial infarction located in arteries with diameters from 2 mm to 4.5 mm.
Features
- iVascular’s proprietary coating technology “TransferWise”. Nanotechnology drop dosage system that yields a multilayer thin coating.
- Improved coating mechanical resistance. Better adhesion to the stent. Homogeneous, durable and flexible formula.
- Triple layer coating (primer + matrix + top) to yield a controlled Sitrolimus eluting profile.
- Fluorinated acrylate polymer with selective cellular activity: Prevents smooth muscle cell deposition while enhancing rendothelization.
- Specific stent design for DES: alternate spiral links and more crowns per turn for a balanced arterial coverage to assure homogeneous drug distribution.
- Outsanding radial force, best in its class.
- Faultless coating after stent expansion
- Ultrathin strut design (75 µm) for clinical safety
- Very low crossing profile and hydrophilic coated shaft for smooth trackability
Specifications
- Stent material: CoCr L605
- Strut thickness: 75 µm – 85 µm depending on stent diameter. Links 70 µm
- % recoil: < 5%
- % shortening at expansion: < 3%
- Drug: Sirolimus 1.4 µg/mm2
- Polymer: Biostable fluorinated acrylate
- Catheter materials: Nylon / Pebax. No latex components.
- Semi-compliant balloon
- Nominal pressure: 9 – 12 atm
- Rated Burst Pressure (RBP): 16 atm
- Average Burst Pressure (ABP): 22 atm
- 2 Pt/Ir radiopaque markers on the catheter delimiting the stent
- Guiding catheter compatibility: 5F
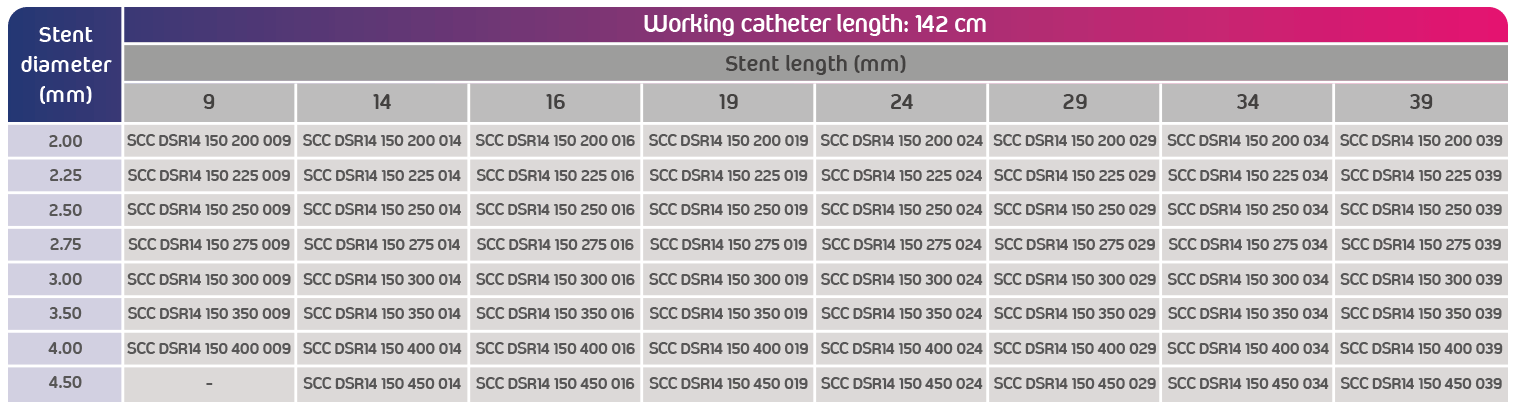
- Catheter’s working length: 142 cm
- Recommended guidewire: 0.014”
- Entry profile: 0.016”
- Wide size range
Clinical Support
Initial preclinical studies are required during the process of improving polymers, platforms, and drug-eluting systems for new coronary stent designs. The objective is to analyze the efficacy and safety of the new drug eluting stent system angiolite compared to conventional BMS in an experimental model with healthy porcine coronary arteries. Angiolite showed less late loss and angiographic restenosis than conventional BMS. Histologically, restenosis and neointimal area were also lower. Safety data showed that while inflammation was lower for angiolite compared to BMS, endothelization stayed at equivalent rates. For further detail of the study check “Rev Esp Cardiol (Engl Ed). 2015 Jul 20” or download the file.
ANCHOR clinical trial: First-in-man assessment of feasibility, exploratory efficacy and clinical performance of angiolite DES utilizing intracoronary optical coherence tomography (OCT) coordinated by Prof. Dr. Josep Rodés-Cabau in Canada. Including 100 patients with de novo epicardial lesion, including those with chronic stable angina, acute coronary syndrome (ACS: defined as non-ST segment elevation myocardial infarction (NSTEMI) or ACS with negative cardiac enzymes). Target lesion must have > 70% stenosis or evidence of ischemia. An adaptive design component of the study randomizes patients to either to either 3-month or 6-month angio/optical coherence tomography follow-up. Clinical follow-up occurs at 30 days, 3 months, 6 months, 12 months and 24 months post PCI. Primary endpoints are 6-month neointimal coverage (n=50) and 6-month neointimal obstruction (n=50). Secondary endpoints are 3-month neointimal coverage (n=25), 3-month neointimal obstruction (n=25), 3 and 6-month malapposition rate (OCT strut-level analysis, n=75), 6-month in-stent angiographic late lumen loss (QCA analysis), 6-month in-stent and in-segment restenosis (QCA analysis), 6-month and 1-year major adverse cardiac events (MACE: CV death, MI, clinical target lesion revascularization (TLR), stent thrombosis).
*Puri R, Otaegui I, Sabate M, et al. Three- and 6-month optical coherence tomographic surveillance following percutaneous coronary intervention with the Angiolite® drug-eluting stent: The ANCHOR study. Catheter Cardiovasc Interv. 2017;00:000–000. https://doi.org/10.1002/ccd.27189
Package Contents
- One sirolimus-eluting coronary stent system comprising the coated stent pre-mounted on its delivery system (balloon catheter). The stent is protected by a sheath and a stylet inserted at the guidewire lumen. The whole system is introduced into a dispenser and in a sterile bag. Finally, the bag with sterile contents is inserted into a secondary foil bag to protect it from light.
- One card with the compliance curve showing the working pressure range.
- One implantation card.
- One leaflet with instructions for use.