Description
Description
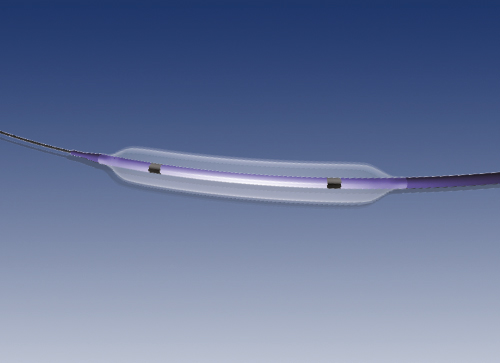
The peripheral stent “Restorer” is made of a Cobalt Chromium alloy called L605. The stent is supplied pre-mounted on a delivery system for its implantation at the lesion to treat by means of expansion of the balloon located at its distal end. The device is designed for percutaneous transluminal angioplasties of peripheral arteries.
The stent is designed to be implanted in different artery diameters through adaptation of its open cell design with alternating connection points.
The stent is manufactured by laser cutting of a metallic tube and then it is submitted to several treatments in order to reach a smooth and glossy surface finish. The stent design is based on circumferential cells linking which are axially interconnected by means of bridging links that provide different longitudinal configurations.
The stent delivery system is a balloon catheter with double lumen configuration and OTW architecture. It features a double lumen tube from the proximal connector to the balloon. One lumen is for guide wire insertion, which will lead the catheter to the lesion, and the other one comprises the inflation channel, which allows contrast liquid to flow to inflate the balloon. The guidewire diameter must not exceed 0.89 mm = 0.035’’.
At the proximal end is located the connector with two entry ports, one for balloon in/deflation and the other one for guidewire passage.
The catheter shaft surface is homogeneously coated with a lubricious silicone based coating to minimize friction and facilitate navigation through vessels.
See IFU for further information. Available to download.
Indications
Restorer is indicated for the treatment of de novo or restenotic atherosclerotic lesions in protected peripheral arteries located under the aortic arch; that is, the iliac artery and the deep femoral artery or proximal femoral artery, and for palliation of biliary tract malignant stenosis with a nominal diameter ranging from 5 and 10 mm.
Features
- Enhanced trackability due to restorer’s unique and flexible strut design featuring nested peaks and helicoidal linkage system
- Increased crossability in difficult lesions thanks to the low strut thickness
- Extraordinary radial force with less metal. Restorer demonstrates excellent vessel support
- Minimized potencial of restenosis due to its optimized metal and ultrasmooth electropolished surface
Specifications
- Stent material: CoCr L605
- Balloon: semi-compliant
- Nominal pressure: 10-12 atm
- Rated Burst Pressure (RBP): 16 atm for up to 7 mm diameter stents, 15 atm for 8 mm diameter stents, and 14 atm for 9 and 10 mm diameter stents
- Radiopaque markers: metallic markers located at both ends of the stent
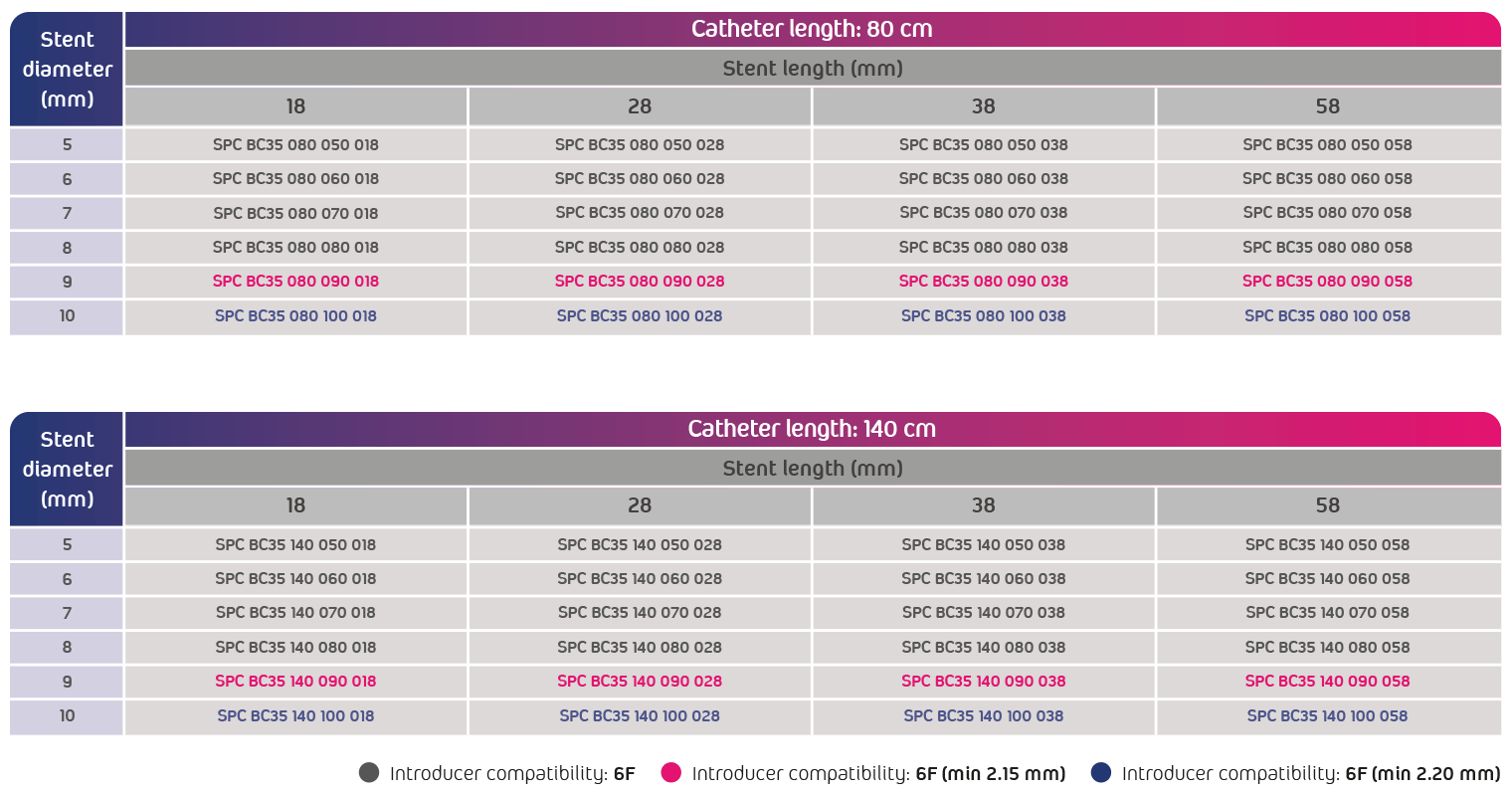
- Introducer compatibility: 6F
- Guide wire compatibility: 0.035’’
- Tip profile: 0.036″
- Deflation time: < 30 s
- Recoil: <6%
- Usable catheter length: 80 cm or 140 cm
- Size range:
Package Contents
- A peripheral stent system consisting on a pre-mounted stent in its delivery system (balloon catheter). The stent is protected by a sheath and introduced in a dispenser and sterile pouch.
- A card with a compliance curve showing the working pressure range.
- An implantation card.
- A leaflet with instructions for use.