Description
Description
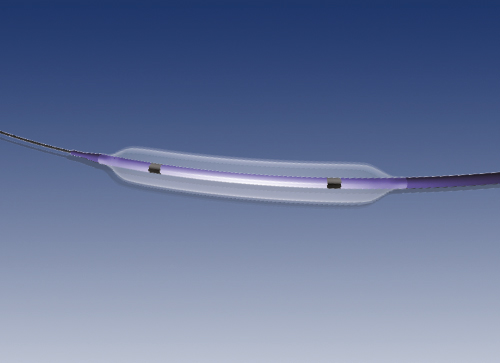
The peripheral iVolution Stent is a stent made out of a nickel/titanium alloy (nitinol). The stent is loaded into the delivery system that will release it at the site of implantation.
The stent is self-expanding, achieving the diameter it has been designed for once it is released from the delivery device. From that moment on, it remains implanted in the artery, exerting a continuous force on the artery wall to stay open.
The stent design is based on a plurality of undulating rings that extend axially without connection bridges forming an open-cell stent.
The metal at the stent ends is less dense in artery coverage, and incorporates a series of radiopaque markers to visualize the stent once expanded.
The stent is made from a nitinol tube that is cut using a laser technique and then expanded to the required final diameter. The surface is then polished to achieve a smooth, shiny finish.
The stent delivery system is a coaxial catheterwith triple sheath design, consisting of:
- A guide wire lumen, with the stent accommodated at the distal end. The distal part of this tube ends in an atraumatic tip that carries a radiopaque marker that defines the distal portion of the stent.
- A stent blocking tube which prevents the stent from moving back at the time of its release. The blocking tube has a distal marker which aligns with the proximal part of the stent, and indicates the position of the stent within the device.
- A retractable sheath that protects and contains the stent. When the sheath is pulled back, the stent is deployed.
- A fixed sheath partially covers the retractable sheath and protects it, so that if the user touches the catheter, doesn’t block the movement of the retractable sheath.
- For the release of the stent, it is necessary to operate the proximal handle of the delivery system. The handle has a locking mechanism that must be disabled to begin the release. The release may be performed slowly, by turning the screw; or it can be performed quickly, by pressing the button and pulling back. The handle is ergonomic and can be used with one hand.
The system ends in a soft, atraumatic tip to avoid damaging the artery during its advance
See IFU for further information. Available to download.
Indications
The peripheral self-expanding stent system iVolution is indicated for the treatment of de novo or restenotic atherosclerotic lesions in peripheral arteries located under the aortic arch and for palliation of biliary tract malignant stenosis with a nominal diameter ranging from 4.5 and 9.5 mm.
Features
- Controlled and precise stent release: triple sheath design, 2 radiopaque markers limiting the stent plus a marker on the moving sheath
- Dual delivery system: scroll for precise slow release, pull-back for quick release.
- Innovative stent design: linkless continuous architecture, open short-cell design, homogeneous radial force, anti-kinking
- Balance between adaptability to the vessel (flexibility) and arterial support (radial force)
Specifications
- Recommended guidewire: 0.035”
- Recommended Introducer: 6F
- Recommended guiding catheter: 8F
- Stent material: nitinol
- Wall thickness: 180 – 190 µm
- Expansion shortening <5%
- Circumferential radial force (at 15% strain): 0.120 mN
- % artery coverage: 15% average
- High vessel adaptability
- Usable catheter lengths: 80 cm or 140 cm
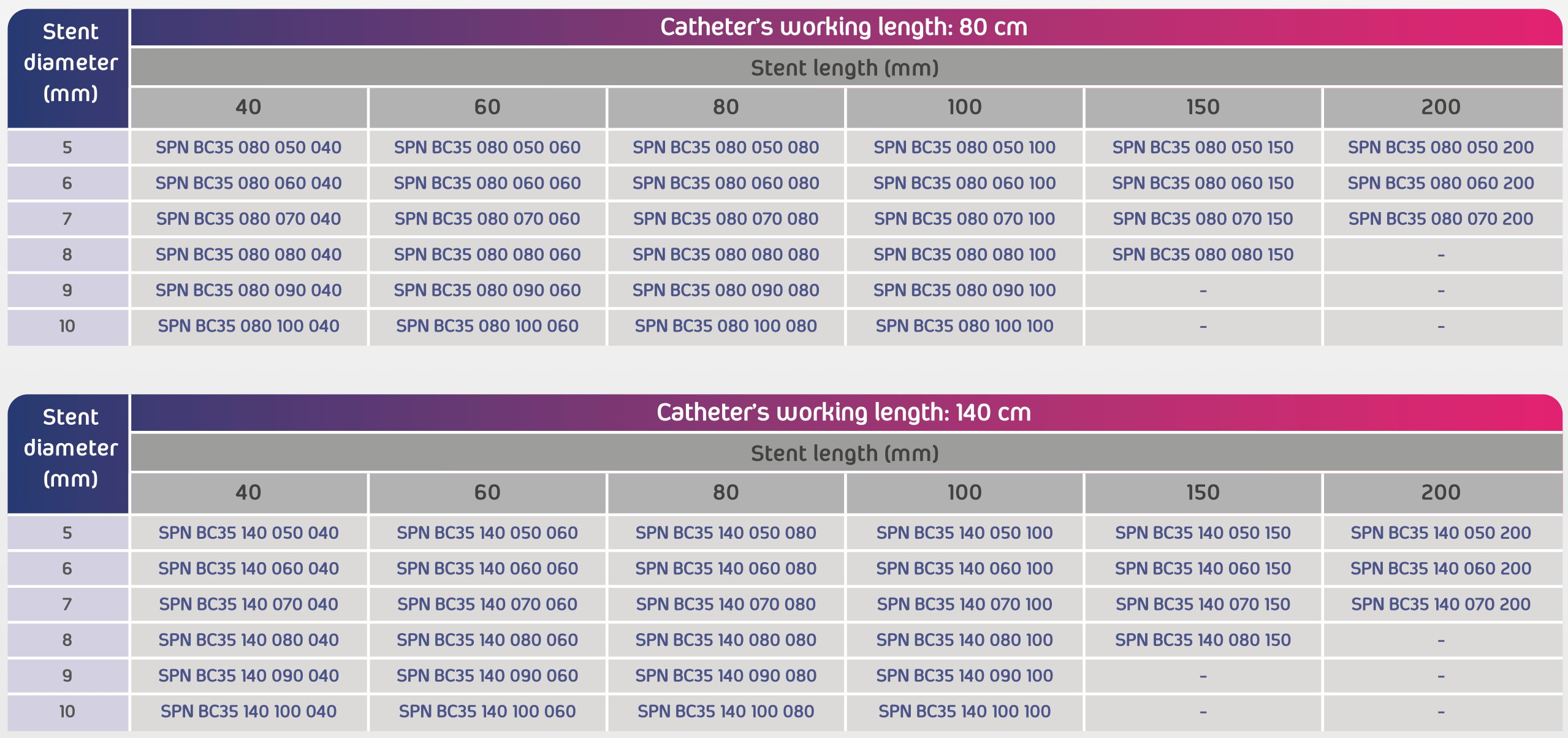
- Size range:
Clinical Support
EVOLUTION trial. The objective of this clinical investigation is to evaluate the short-term (up to 12 months) outcome of treatment by means of the self-expanding iVolution nitinol stent in symptomatic (Rutherford 2-4) femoropopliteal arterial stenotic or occlusive lesion of ≤ 15 cm. The study involves 120 patients and is coordinated by Dr. Marc Bosiers in Belgium. Primary endpoint is the Primary Patency at 12 months, defined as freedom from >50% restenosis at 12 months as indicated by duplex ultrasound in the target vessel with no reintervention. Secondary endpoints are: Primary Patency rate at 1 & 6-month follow-up, Technical success defined as the ability to cross and stent the lesion to achieve residual angiographic stenosis no greater than 30% and residual stenosis less than 50% by duplex imaging, Freedom from Target Lesion Revascularization (TLR) at 1, 6 & 12-month follow-up, improvement of Rutherford stage in one class or more at 1, 6 & 12-month follow-up and serious adverse effects.
Package Contents
- One peripheral self-expanding stent mounted on its delivery system. Packed in a blister and bag to protect sterility.
- One implantation card.
- One leaflet with instructions for use.