Description
Description
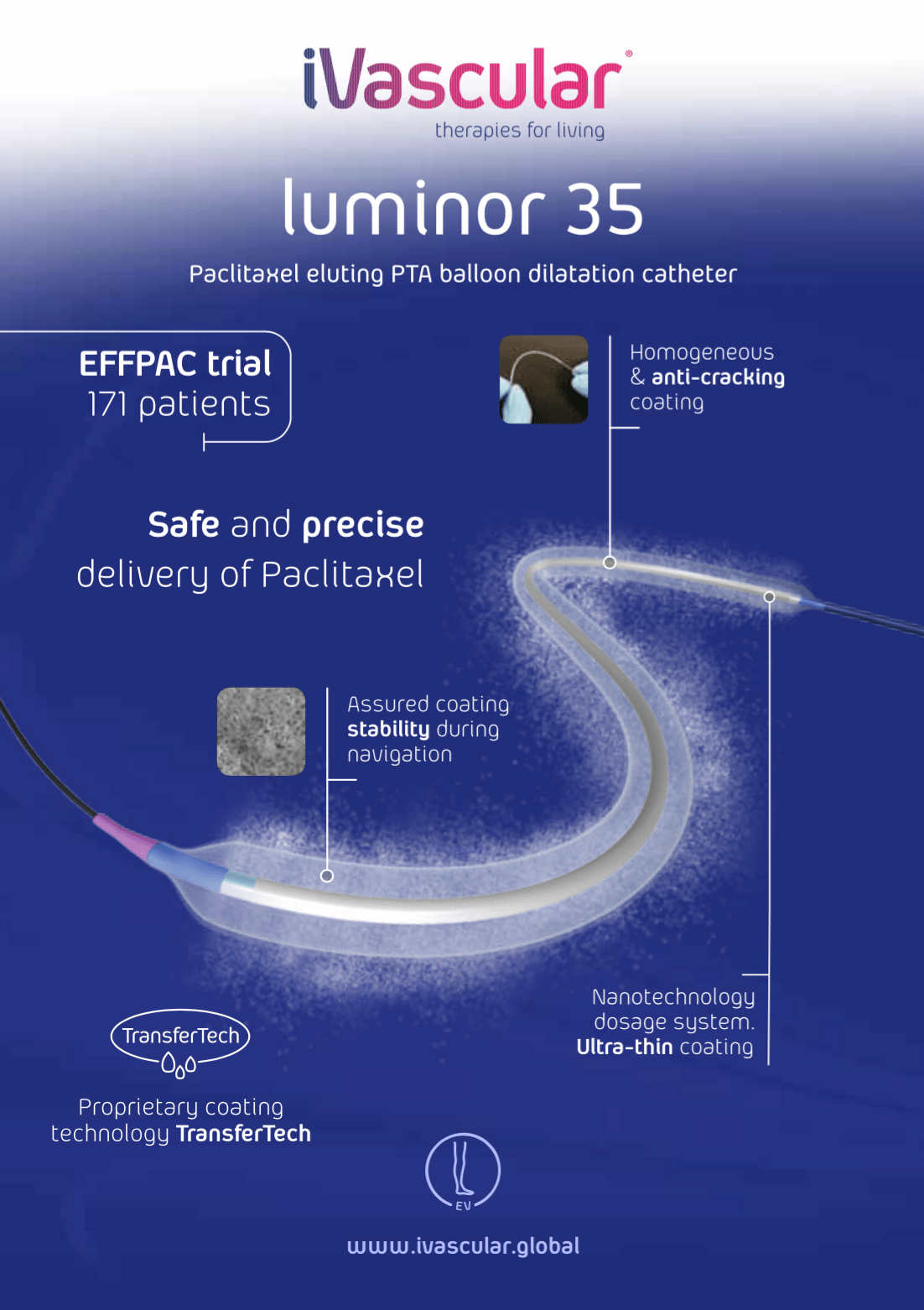
The Paclitaxel eluting balloon luminor 35 is a double lumen catheter from the connector to the tip (also called over-the-wire, OTW) designed for percutaneous transluminal angioplasties of large peripheral arteries.
The guidewire runs into the circular-largest lumen while the elliptic-smallest lumen is intended for contrast medium flow. The maximum diameter of the guide wire must not exceed 0.89 mm = 0.035 inches.
On the catheter’s distal part, just before the tip, there is the balloon (inflatable segment) that will dilate the artery upon inflating by means of infusion of contrast fluid inside it. The connector is Y-shaped and it has two entry ports:
- The straight port is intended for guidewire passage.
- The side port allows contrast medium flow to dilate the balloon
The balloon is coated with a homogeneous mixture of Paclitaxel, a derivative of Taxol, and a physiologically innocuous matrix, the excipient. Drug’s dose is 3 µg/mm2of balloon surface and it is intended to avoid cellular proliferation, consequently decreasing re-intervention rate.
Drug is released from the balloon by means of a rapid inflation so that a high dose is released in a very short period of time. In order to assure a sufficient dosage of Paclitaxel onto the arterial wall, inflation process must last from 30 seconds to 1 minute. Dilation of the lesion can be optimized by using longer inflation times at operator’s discretion.
The balloon is designed to reach different diameters at different pressures, as predicted by the compliance curve included on the primary packaging.
Two radiopaque markers of Pt/Ir alloy are located at each end of the balloon in order to mark its length and help the user to see the catheter while navigating inside the patient.
At the distal end there is the tip, made from a very soft polymer and rounded in a non-traumatic shape in order to avoid damaging the arterial wall while navigating.
The shaft of the catheter is coated with a proprietary hydrophilic formula in order to minimize friction
See IFU for further information. Available to download.
Indications
The device is indicated for dilation of stenosis located in the iliac, femoral, ilio-femoral, popliteal, infrapopliteal and renal arteries, as well as for the treatment of obstructive lesions of arteriovenous fistulas, whether original or artificial, with a reference diameter balloon from 5 to 7 mm and lengths from 20 to 150 mm. It is also indicated for stent post-dilation in the peripheral vascular system.
Features
- iVascular’s proprietary coating technology “Transfertech”. Nanotechnology drop dosage system that yields a multilayer thin coating.
- Improved coating mechanical resistance. Better adhesion to the balloon
- Homogeneous and precise Paclitaxel concentration of 3 µg/mm2 on the balloon for an effective treatment of the whole injury
- Microcrystalline coating for faster drug absorption rate. Rapid release of Paclitaxel with long-term efficacy.
- Segmented catheter design for excellent pushability and trackability. Balanced transition of forces. Enhanced dual lumen shaft design. Anti-kinking performance.
- Outstanding crossing profile thanks to the 6 wings folding and 3 wings re-folding for easy extraction through the sheath.
- Very short balloon deflation rates.
- Wide balloon size range (150mm balloons)
- Proprietary hydrophilic coating “Hydrax” on the shaft of the catheter to improve trackability
Specifications
- Catheter materials: Nylon/Pebax (the product does not contain latex components)
- Drug: 3 µg/mm2 Paclitaxel
- Excipient: Organic ester. Lipophilic, biocompatible and biodegradable.
- Balloon: Semi-compliant (10 from nominal pressure to RBP)
- Nominal Presure: 6/7 atm
- Rated Burst Pressure (RBP): 16 atm
- Average Burst Pressure (AVP): 23 atm
- Recommended guidewire: 0.035”
- Crossing profile: 0.037”
- Introducer compatibility: 5F (d=5mm), 6F (d=6mm, d=7mm)
- Deflation time: < 5s
- 2 Platinum / Iridium radiopaque markers embedded at the guidewire lumen
- Working catheter lengths: 80 cm and 140 cm
- Available references:

Clinical Support
Paclitaxel-eluting balloons have shown antiproliferative efficacy in the treatment and prevention of restenosis. Nevertheless, not all available devices are equally effective, which makes it interesting to compare results in a preclinical swine model (overexpansion 1.2 to 1.0). In this model, iVascular’s luminor DEB significantly reduced in-stent restenosis compared with the control balloons. Study published in the Spanish Journal of Cardiology (Rev Esp Cardiol. 2014;67:456-62 – Vol. 67 Num.06) and available for download on website.
EffPac trial: Phase III multicenter randomized controlled trial to assess the EFFectiveness of PAClitaxel coated Luminor® balloon catheter in stenotic or occlusive lesions (Rutherford 2-4) between 5 and 15 cm (TASC II A and B ) in the superficial femoral artery compared to non-coated plain old angioplasty balloon (POBA) catheter. 172 subjects are being treated with either DEB or POBA in 11 study centers in Germany under coordination of Prof. Dr. Ulf Teichgräber. Primary endpoint is the late lumen loss at 6 and 12 months. Secondary end-points are target lesion revascularization, Quality of life, amputation rate, mortality, change of Rutherford stage, ankle-brachial index and walking distance.
LUMINOR Registry: An observational, prospective and multicentre study with single-arm treatment for native stenotic or occlusive lesions or in-stent stenosis of the femoro-poplietal (FP) and below the knee (BTK) vessels. The objective is to evaluate the performance of luminor drug-eluting balloons in terms of primary patency and freedom of serious adverse events. A maximum of 250 Rutherford 2-5 cases will be recruited following an intention to treat basis.
Since Q3 2014 until Q2 2016, 215 cases with 252 lesions (121 CTO and 131 stenosis) have been included and monitored. Those were split as 154 FP and 86 BTK vessels treated. 12 cases combined both segments. It is important to emphasize that 72% of patients were classified as Rutherford 4 or 5 . Technical success was achieved in 94,9% of the cases. Bailout stenting was necessary in 15 lesions (6%).
30-day-mortality was 1,9%. Until now, 145 patients have reached 6 months of follow-up and 108 patients 1 year.
At 1 year, primary patency was 94,0%, freedom from TLR 96,2%, freedom from major amputation 89,8% and survival 90,4%.
Package Contents
– A paclitaxel-eluting dilatation balloon catheter OTW, covered by a protective shaft over the balloon and a protection stylet on the guidewire lumen. All the set is introduced in a circular dispenser to avoid damaging the catheter, and it is packed into a sterile bag.
– One card with the compliance curve showing the nominal inflation pressure and the recommended maximum pressure
– One leaflet with instructions for use