Description
Description
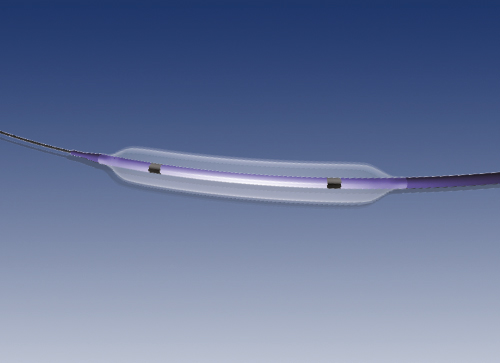
iVascular Architect Coronary Stent is a stent made of a Cobalt Chromium alloy called L605. The stent is supplied pre-mounted on a delivery system for its implantation at the coronary lesion to treat, by means of expansion of the balloon located at the distal end of the catheter.
The stent is manufactured by laser cutting of a metallic tube and then it is submitted to several treatments in order to reach a smooth and glossy surface finish. The stent design is based on circumferential cells linking which are axial interconnected means of connecting links that provide different stent longitudinal configurations. On the other hand, the number of radial cells adjustment allows stent expanding to different diameters. It is based on an open-cell design.
The stent delivery system consists on a rapid-exchangeballoon catheter. The catheter comprises an inflatable balloon at the distal part. The balloon has different diameters and lengths to fit with the different stent configurations. When the balloon is inflated, it dilates the stent and deploys it against the artery. Afterwards, the balloon is deflated and removed, and the stent remains permanently implanted.
There are marks on the catheter shaft to help the user to calculate the position of the catheter as it is advanced through the guiding catheter without fluoroscopy, so that, when the last mark disappears, the catheter is already near to the tip of the guiding catheter and about to enter the artery.
The distal part of the catheter is coated with a durable hydrophilic coating that minimizes friction and improves catheter trackability.
The maximum guide wire passage diameter must not exceed 0.36mm = 0.014”. Useful length of the catheter is 142cm, while the total length is 150cm.
See IFUs for further information. Available to download.
Indications
The device indication is increasing the internal diameter of an artery with the aim of improving blood flow in the following cases:
- Patients with symptomatic ischemic heart disease due to “de novo” stenotic and re-stenotic lesions located in arteries with diameters from 2 mm to 4.5 mm.
- Patients with ischemic disease at coronary bypass grafts, including saphene vein grafts.
- Patients with occlusive disease due to acute myocardial infarction located in arteries with diameters from 2 mm to 4.5 mm.
Features
- Smooth balanced transition of forces for high proximal pushability and distal flexibility
- Extraordinary radial force with less metal. Architect demonstrates excellent vessel support
- Enhanced trackability due to its unique flexible strut design
- Increased crossability for difficult lesions
- Minimized potential of restenosis due to its optimized metal and ultrasmooth electropolished surface
Specifications
- Stent material: CoCr L605
- Balloon material: nylon/pebax. It does not contain latex components
- Balloon: semi-compliant
- Nominal Pressure: 11 atm
- Rated Burst Pressure (RBP): 16 atm
- Average Burst Pressure (ABP): 22 atm
- Radiopaque markers: metallic markers located at both ends of the stent
- Guiding catheter compatibility: 5F
- Useful Catheter length: 142 cm
- Catheter diameter: 2F proximal, 2.6F middle and 2.2F distal
- Guide wire port- tip length: 25 cm
- Recommended guide wire: 0.014’’
- Deflation time: 3s (average)
- Recoil: <5%
- Available lengths: 9 to 39 mm
- Size range:

Package Contents
- One coronary stent system comprising the stent pre-mounted on its delivery system (balloon catheter).
- The stent is protected by a sheath and a stylet inserted at the guidewire lumen. The system is inserted into a dispenser and in a sterile bag.
- One compliance curve card showing the working pressure range.
- One implantation card.
- One leaflet with instructions for use.