Description
Description
The Paclitaxel-eluting balloon “essential” is a rapid exchange catheter (also known as RX) designed for percutaneous transluminal angioplasties of coronary arteries.
The body of the catheter features a combination of a single lumen at the proximal end and a dual lumen at the distal end.
- The single lumen at the proximal end allows contrast liquid to pass through between the proximal luer-lock connector and the balloon during in/deflation.
- The coaxial dual lumen at the distal end has two functions:
- A lumen to carry the contrast liquid used to inflate the balloon, connected to the proximal lumen
- A lumen for the guidewire to facilitate and allow the passage of the catheter towards and through the stenosis to be dilated. The maximum diameter of the guidewire must not exceed 0.36 mm = 0.014 inches.
On the catheter’s distal part, just before the tip, there is the balloon (inflatable segment) that will dilate the artery upon inflating by means of infusion of contrast fluid inside it.
The balloon is coated with a homogeneous mixture of Paclitaxel, a derivative of Taxol, and a physiologically innocuous matrix, the excipient. The drug’s dose is 3 µg/mm2 of balloon surface and it is intended to avoid cellular proliferation, consequently decreasing re-intervention rate.
The drug is released from the balloon by means of a rapid inflation so that a high dose is released in a very short period. In order to assure a sufficient dosage of Paclitaxel onto the arterial wall, inflation process must last from 30 seconds to 1 minute. Dilation of the lesion can be optimized by using longer inflation times at operator’s discretion.
The balloon is designed to reach different diameters at different pressures, as predicted by the compliance curve included on the primary packaging.
Two radiopaque markers are located at each end of the balloon in order to mark its length and help the user to see the catheter while navigating inside the patient.
The catheter ends in a cone-shaped tip
See IFU for further information. Available to download.
Indications
Dilatation of stenotic portions in coronary arteries or stenosis after bypass grafts which replace coronary arteries. Small vessels can also be treated, as well as residual stenosis after balloon treatment or endoprosthesis, and also pre/post-dilatation of endovascular coronary prosthesis, with the aim of improving myocardial perfusion.
Specifications
- Catheter materials: Nylon/Pebax (the product does not contain latex components)
- Drug: 3 µg/mm2 Paclitaxel
- Excipient: Organic ester. Lipophilic, biocompatible and biodegradable.
- Balloon: Semi-compliant (10-15% from nominal pressure to RBP)
- Nominal Presure: 6 atm
- Rated Burst Pressure (RBP): 16 atm
- Average Burst Pressure (AVP): 20 atm
- Recommended guidewire: 0.014”
- Tip profile: 0.016”
- Crossing profile: from 0.021’’ up to 0.027’’
- Introducer compatibility: 5F for all diameters (6F for kissing balloon technique)
- Deflation time: 3s average
- 2 tungsten-based flexible polymeric radiopaque markers
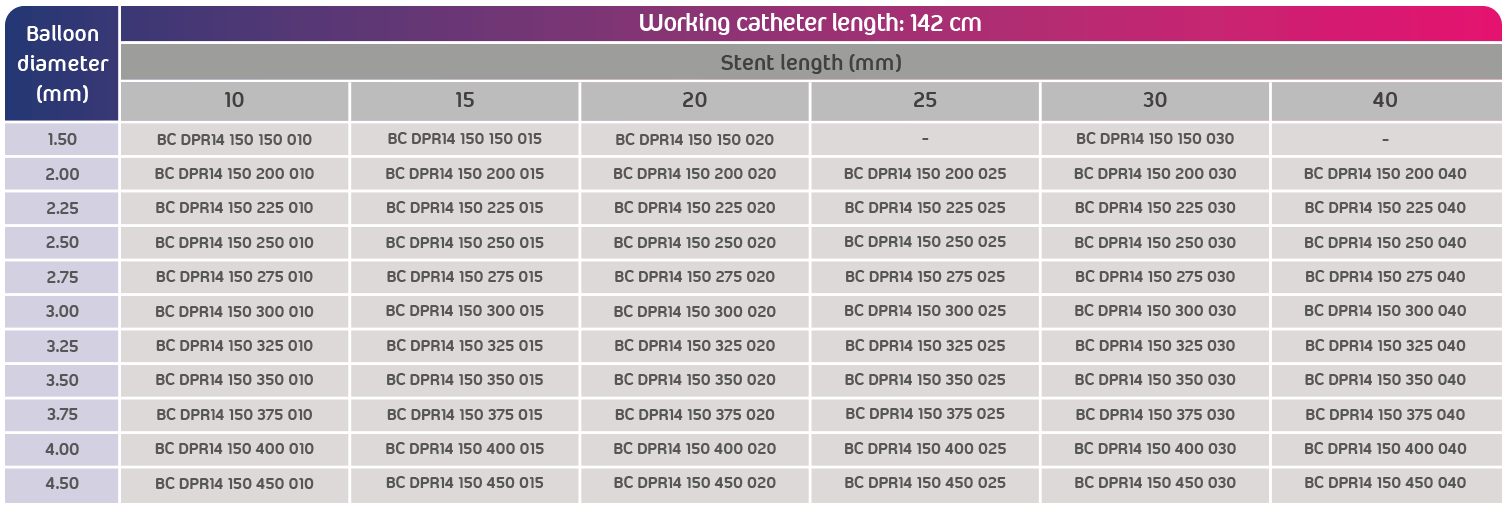
- Usable catheter length: 142 cm
- Available references
Clinical Support
Paclitaxel-eluting balloons have shown antiproliferative efficacy in the treatment and prevention of restenosis. Nevertheless, not all available devices are equally effective, which makes it interesting to compare results in a preclinical swine model (overexpansion 1.2 to 1.0). In this model, iVascular’s luminor 14 DEB significantly reduced in-stent restenosis compared with the control balloons. Study published in the Spanish Journal of Cardiology (Rev Esp Cardiol. 2014;67:456-62 – Vol. 67 Num.06) and available for download on website.
Package Contents
- A paclitaxel-eluting dilatation balloon catheter RX, covered by a protective shaft over the balloon and a protection stylet on the guidewire lumen. All the set is introduced in a circular dispenser to avoid damaging the catheter, and it is packed into a sterile bag.
- One card with the compliance curve showing the nominal inflation pressure and the recommended maximum pressure
- One leaflet with instructions for use
Features
- iVascular’s proprietary coating technology “TransferTech”. Nanotechnology drop dosage system that yields a multilayer thin coating.
- Improved coating mechanical resistance. Better adhesion to the balloon
- Homogeneous and precise Paclitaxel concentration of 3 µg/mm2 on the balloon for an effective treatment of the whole injury
- Microcrystalline coating for faster drug absorption rate. Rapid release of Paclitaxel with long-term efficacy.
- Segmented catheter design for excellent pushability and trackability. Balanced transition of forces. Anti-kinking performance.
- Tungsten-based polymeric radiopaque markers for higher tip flexibility. Outstanding deliverability.
- Hydrophilic durable coating (HYDRAX) guarantees sustainable performance and control throughout the procedure
- Exceptional lesion crossing capability due to its tip profile
- Very short balloon deflation rates.
- Wide balloon size range.